Introduction:
Alterations of core binding factors (CBF), Runx1 and CBFβ are frequent mutational targets in acute myeloid leukemia (AML). Chromosomal translocations t(8;21)(q22;q22) and inv(16)(p13q22), creating the fusion proteins RUNX1-ETO and CBFβ-MYH11 respectively, account for 15% and thus the largest sub-group of AML called CBF-AML. CBF oncogenes induce global changes in chromatin structure and gene regulation, which lead to differentiation blockade. A critical leukemic event could be the inactivation of PU.1 transcription factor. Normal myeloid differentiation needs PU.1 levels to increase, failure to do so leads to a stop of differentiation and AML development. In contrast, T-cell differentiation requires PU.1 to be completely switched off. The exact mechanism of PU.1 suppression, physiological for T-lymphopoiesis or pathological for leukemia, remains elusive.
Results:
We assessed the activation of the PU.1 locus throughout human hematopoietic differentiation stages using the assay for transposase-accessible chromatin with high-throughput sequencing (ATAC-seq) and reverse-transcription quantitative polymerase chain reaction (RT-qPCR). Interestingly, we observed high accessibility of a previously identified antisense promoter (AsPr) in intron 3 and antisense transcript (asRNA) expression during early lymphopoiesis which preceded locus shutdown in T cells. The ratio of AsPr/PrPr accessibility and of antisense/sense transcription clearly indicated cellular fate during hematopoiesis (Figure 1A). T-lymphoid differentiation was related to the timely expression of RUNX transcription factors. RUNX1, RUNX3, and the CBF fusions RUNX1-ETO and CBFβ-MYH11 were capable to transactivate PU.1 AsPr. In CBF-AML patient samples we strikingly found elevated asRNA/mRNA ratios compared to normal karyotype AML or healthy CD34+ cells (Figure 1B) and increased AsPr/PrPr ratios unsing DNaseI-seq data in RUNX1-ETO AML patients.
Functionally we found that PU.1 asRNA depletion in t(8;21) xenografted immune-deficient (NOD/SCID) mice restored a normal survival (Figure 1C) demonstrating that PU.1 antisense transcripts are required for CBF leukemia outgrow in vivo. To further dissect the mechanism of how CBFs could drive PU.1 antisense transcription we applied active RNA polymerase mapping (PRO-seq) and chromatin accessibility (ATAC-seq) and found a shift from PU.1 antisense to sense transcription after RUNX1-ETO depletion.
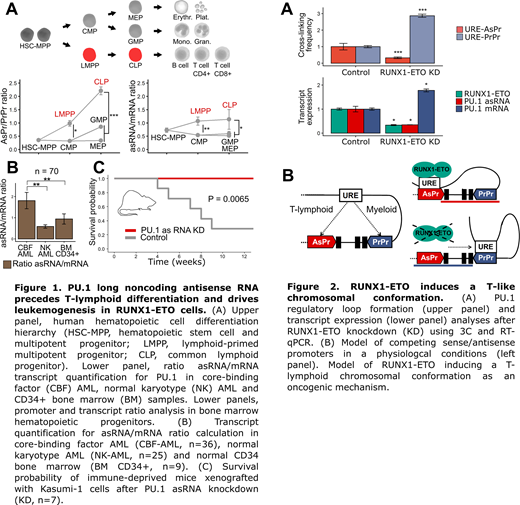
Using chromosomal conformation capture sequencing (3C, Hi-C and CHiC) in T-lymphoid, myeloid and RUNX1-ETO cells combined with transcript quantification we observed that competitive interaction of an upstream enhancer with the proximal or the antisense promoter are at the heart of differential PU.1 expression during myeloid and T-cell development (Figure 2A). Leukemic CBF fusions thus utilize a physiologic mechanism employed by T-cells to decrease sense PU.1 transcription (Figure 2B).
Conclusion:
The data suggest that silencing transcription factor PU.1 is an active process that requires a specific chromosome formation that is induced by CBF fusions. Sense/antisense promoter competition represents a crucial functional switch for gene expression perturbation by oncogenes and provide a potential strategy for future precise therapeutic targeting of oncogene-induced chromatin remodeling.
Valent:Allcyte GmbH: Research Funding; Pfizer: Honoraria; Cellgene: Honoraria, Research Funding. Staber:Roche: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; msd: Consultancy, Honoraria; Celgene/ BMS: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal